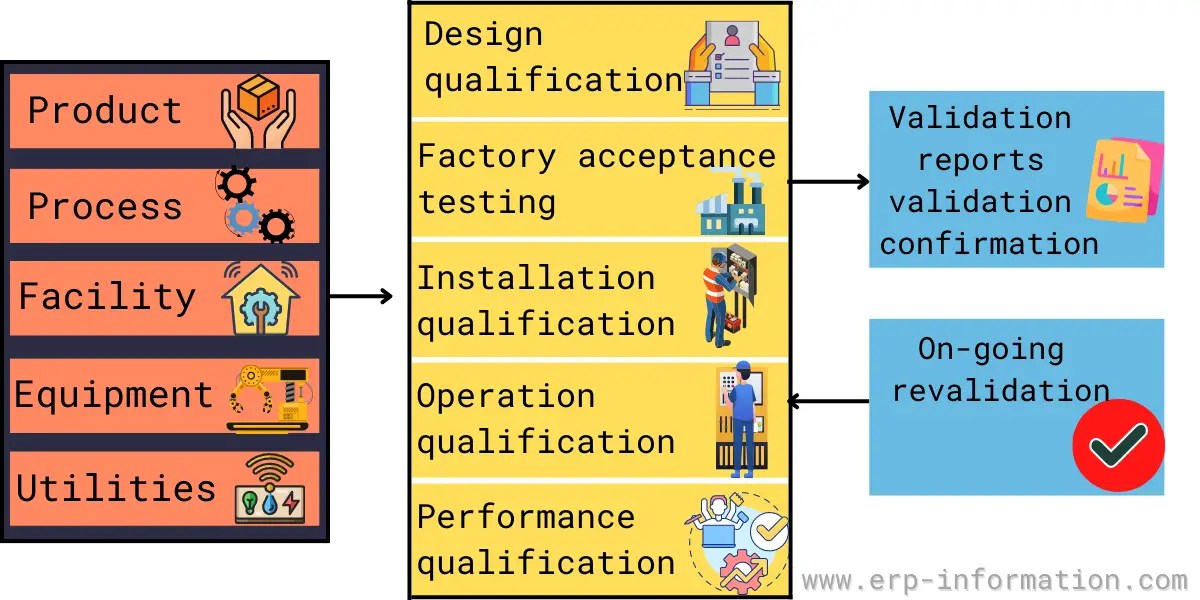
Validation Master Plan Template - Web three (3) options to create a validation master plan. Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation. Web fda quality systems regulations. Web this article can help you understand who is responsible for preparing the validation master plan, the stages of. Web buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. Web at the core of the validation process is a fundamental document known as a validation master plan (vmp). You can create a great protocol, using a template. Web learn the definition, functions, and critical components of a validation master plan (vmp) for pharmaceutical manufacturing. The purpose of this guideline is to provide guidance on the.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
You can create a great protocol, using a template. Web three (3) options to create a validation master plan. Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation. Web fda quality systems regulations. Web this article can help you understand who is responsible for preparing the validation master plan, the.
Overview of the Validation Master Plan PresentationEZE
Web this article can help you understand who is responsible for preparing the validation master plan, the stages of. The purpose of this guideline is to provide guidance on the. Web three (3) options to create a validation master plan. Web fda quality systems regulations. You can create a great protocol, using a template.
Validation Master Plan Template
Web learn the definition, functions, and critical components of a validation master plan (vmp) for pharmaceutical manufacturing. Web buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. You can create a great protocol, using a template. Web fda quality systems regulations. Web a validation master plan (vmp) is a documented.
Validation Master Plan Bio Chem Shop
Web this article can help you understand who is responsible for preparing the validation master plan, the stages of. Web learn the definition, functions, and critical components of a validation master plan (vmp) for pharmaceutical manufacturing. Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation. Web at the core of.
What is Validation Master Plan? (Template, Examples)
You can create a great protocol, using a template. Web this article can help you understand who is responsible for preparing the validation master plan, the stages of. Web buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. Web fda quality systems regulations. Web at the core of the validation.
Validation Master Plan (VMP) Downloadable Interactive Template.
You can create a great protocol, using a template. Web at the core of the validation process is a fundamental document known as a validation master plan (vmp). The purpose of this guideline is to provide guidance on the. Web fda quality systems regulations. Web learn the definition, functions, and critical components of a validation master plan (vmp) for pharmaceutical.
Validation Master Plan Template
Web buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. The purpose of this guideline is to provide guidance on the. Web learn the definition, functions, and critical components of a validation master plan (vmp) for pharmaceutical manufacturing. Web at the core of the validation process is a fundamental document.
Validation Master Plan Egnyte
You can create a great protocol, using a template. Web learn the definition, functions, and critical components of a validation master plan (vmp) for pharmaceutical manufacturing. Web at the core of the validation process is a fundamental document known as a validation master plan (vmp). The purpose of this guideline is to provide guidance on the. Web buy a fully.
Web buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. You can create a great protocol, using a template. Web fda quality systems regulations. Web at the core of the validation process is a fundamental document known as a validation master plan (vmp). Web learn the definition, functions, and critical components of a validation master plan (vmp) for pharmaceutical manufacturing. Web three (3) options to create a validation master plan. Web this article can help you understand who is responsible for preparing the validation master plan, the stages of. Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation. The purpose of this guideline is to provide guidance on the.
You Can Create A Great Protocol, Using A Template.
Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation. Web three (3) options to create a validation master plan. Web buy a fully editable and compliant document template for recording the schedule and status of validations for medical devices. Web this article can help you understand who is responsible for preparing the validation master plan, the stages of.
Web At The Core Of The Validation Process Is A Fundamental Document Known As A Validation Master Plan (Vmp).
Web learn the definition, functions, and critical components of a validation master plan (vmp) for pharmaceutical manufacturing. The purpose of this guideline is to provide guidance on the. Web fda quality systems regulations.